Table of contents
Browse categories
Browse authors
 AB
ABAlberto Boffi
 AL
ALAlessia Longo
 AH
AHAl Hoge
 AB
ABAljaž Blažun
 BJ
BJBernard Jerman
 BČ
BČBojan Čontala
 CF
CFCarsten Frederiksen
 CS
CSCarsten Stjernfelt
 DC
DCDaniel Colmenares
 DF
DFDino Florjančič
 EB
EBEmanuele Burgognoni
 EK
EKEva Kalšek
 FB
FBFranck Beranger
 GR
GRGabriele Ribichini
Glacier Chen
 GS
GSGrant Maloy Smith
 HB
HBHelmut Behmüller
 IB
IBIza Burnik
 JO
JOJaka Ogorevc
 JR
JRJake Rosenthal
 JS
JSJernej Sirk
 JM
JMJohn Miller
 KM
KMKarla Yera Morales
 KD
KDKayla Day
 KS
KSKonrad Schweiger
Leslie Wang
 LS
LSLoïc Siret
 LJ
LJLuka Jerman
 MB
MBMarco Behmer
 MR
MRMarco Ribichini
 ML
MLMatic Lebar
 MS
MSMatjaž Strniša
 ME
MEMatthew Engquist
 ME
MEMichael Elmerick
 NP
NPNicolas Phan
 OM
OMOwen Maginity
 PF
PFPatrick Fu
 PR
PRPrimož Rome
 RM
RMRok Mesar
 RS
RSRupert Schwarz
 SA
SASamuele Ardizio
 SK
SKSimon Kodrič
 SG
SGSøren Linnet Gjelstrup
 TH
THThorsten Hartleb
 TV
TVTirin Varghese
 UK
UKUrban Kuhar
Valentino Pagliara
 VS
VSVid Selič
 WK
WKWill Kooiker
Emergency Ventilator Testing To Aid Hospitals in Coronavirus Crisis

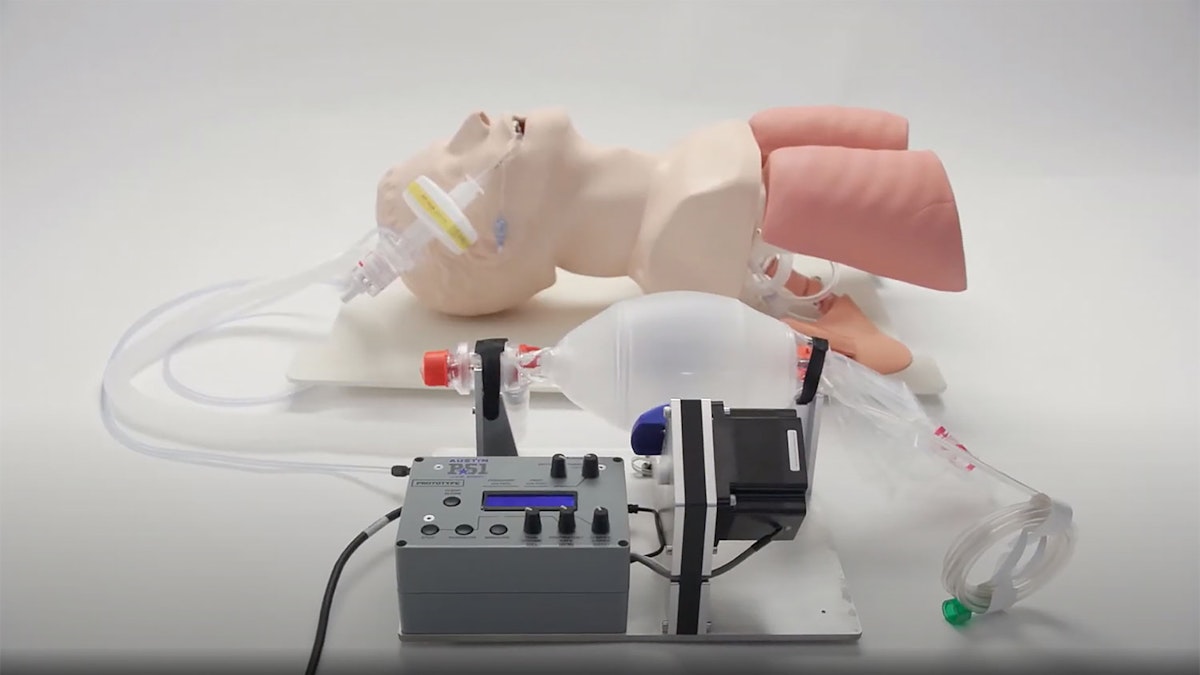
Sisu, a U.S. company dedicated to industrial automation systems, is currently designing and manufacturing the Austin P51, a low-cost ventilator to aid hospitals and medical professionals in the current worldwide shortage. Dewesoft was given the opportunity and was proud to work with SISU in setting up a production line for emergency hospital ventilators.
For most western countries, when the year 2020 began it seemed like a typical new year; the economy was flourishing, businesses were focusing on company goals, individuals were planning vacations and children were dreaming about their upcoming summer break. Little did we know that our generation was on the brink of an unparalleled crisis and that society would be changed forever.
Like ripping off a band-aid, governments around the world announced unprecedented shutdowns with incomprehensive financial and social consequences in attempts to stay ahead of what will never be forgotten as COVID-19.
The devastation of this outbreak varied between countries with many contributing factors including airport traffic, shipyard traffic, tourism, social mannerisms, etc. with little time for preparation. With countries looking at each other for answers, commonalities, and research to help one another, a unanimous, worldwide shortage of key medical equipment quickly became apparent.
Perhaps the most crucial medical device to combat and save lives during this crisis was determined to be the ventilator as COVID-19 became known to attack the respiratory system. In response to this quickly apparent shortage, public announcements of age limits for receiving ventilators streamed to the media like something from a horror film with society looking desperately for answers.
Just as historical wartimes called for individual countries to manufacture vehicles, weapons, and munitions, this outbreak called for humanity to come together to combat the COVID-19 outbreak through emergency production and R&D of medical equipment.
Dewesoft was given the opportunity and was proud to work with SISU in setting up an emergency ventilator production line, called the AUSTIN P51 ventilator.
Sisu and the Austin P51 ventilator
The company Sisu, located in Austin, Texas takes on projects in industrial automation. Product development of industrial machines and turn-key systems are produced for semiconductor, food production, oil and gas, renewable energy, medical, and manufacturing industries. The company serves customers in the United States and around the world and is a Kuka Robotics System Partner and an alliance partner with National Instruments.
The AUSTIN P51 is designed with the intention to be simple to understand and use for all medical professionals. There are two models of the ventilator going into production, an open frame model for more stable and clean environments such as a hospital as well as a highly portable, ruggedized model, for mobile applications.
More information regarding this ventilator is summarized here:
Dewesoft USA received a call on Friday, April 3rd, 2020 during the government-issued quarantine explaining that SISU was trying to validate the proper size for the stepper motor to be used in the AUSTIN P51 ventilator and was looking for assistance.
SISU, stepping up and pushing the limits of emergency medical R&D and production, needed to quickly set up a test correlating lung pressure to the torque of the stepper motor when the adjacent paddles squeeze the self-inflating bag. Air is then driven through a series of pressure regulators and a HEPA filter that can assist or support the human lungs when compromised. With SISU targeting the manufacturing of 100,000 ventilators on a very rigorous timeline, they simply did not have time to program a test to acquire and analyze this data with their current data acquisition system.
Dewesoft’s dynamic and easy-to-use data acquisition hardware and software platform proved to be the fast solution SISU was searching for. Dewesoft personnel was able to respond immediately, arriving at SISU’s facility in Round Rock, TX on Saturday, April 4th to assist SISU in setting up this test.
Dewesoft was able to integrate into their existing setup and sensors while providing results in just a couple of hours. The immediate response and acquired data helped SISU maintain their rigorous timeline. The results of this test were used to validate their stepper motor, design, and computational models. This fast turnaround was vital for keeping their production goals alive in response to the COVID-19 outbreak.
Measurement procedure and setup
The Dewesoft data acquisition platform was incredibly simple to directly integrate into SISU’s already existing setup. A SIRIUS DAQ system with SIRIUS-STG amplifiers was used in conjunction with their torque cell and pressure transducer. Using a DSUB9 connector with screw terminals and the pinouts provided by Dewesoft upon setting up the amplifiers, the sensors were wired up and working in minutes.
Setting up the Torque Cell:
Setting up the Pressure Transducer:
Hardware test setup:
After configuring the torque and pressure sensors, the software was then configured for the engineers to see the real-time data and statistics of the measurements, in order to evaluate the performance of the stepper motor. Furthermore, the incorporation of their conceptual model and video was an additional benefit in this analysis. The following image shows the real-time data that the engineers were able to see to obtain their conclusions regarding the stepper motor design.
Software test setup:
Conclusion
The world hopes to look back on this day and conclude that modern medicine, the age of communication, extreme procedures, and companies’ innovative ideas with “can-do” attitudes were adequately used to combat the COVID-19 virus and prevent history from repeating itself with a worldwide outbreak similar to the Spanish Flu, Black Plague or many others which proved to stunt the growth of humanity.
Here at Dewesoft, we are simply doing our part in taking every possible effort to serve and assist our medical professionals and partners in society who have come together, not to focus on business goals, but to focus on society’s needs to get through this crisis. We are proud of our employees, customers and respective countries in how we have reacted to stay-at-home ordinances and appropriately responded to essential businesses as called upon.
In this case, this is simply a story of a joint effort from a series of companies and individuals coming together to combat a worldwide pandemic. Dewesoft solutions are known for having an exceptional balance of simplicity without sacrificing capability. In this case, it was merely this combination along with the speed in which the test was set up and data was acquired that was needed to maintain this incredibly important timeline.
The biomedical industry is a rapidly growing field and over the past couple of years, Dewesoft has been increasingly involved in this industry. With the threatening COVID-19 situation upon us, we hope to assist our partners in any way possible while making the best of the situation and strengthening our relationships through our dedicated customer service.
Read also:
